Blood flow reduction in neurodegenerative disease
our research focus
Cerebral blood flow (CBF) reductions are a common symptom associated with neurodegenerative disease, which appears early in disease progression, and in the case of Alzheimer’s disease correlate with the severity of cognitive impairment.
My research program will aim to understand the mechanisms leading to blood flow reductions in mouse models of neurodegenerative disease. To achieve this, we will use in vivo multiphoton imaging, which allows us to follow the development and the progression of these reductions during aging in mouse models of dementia.
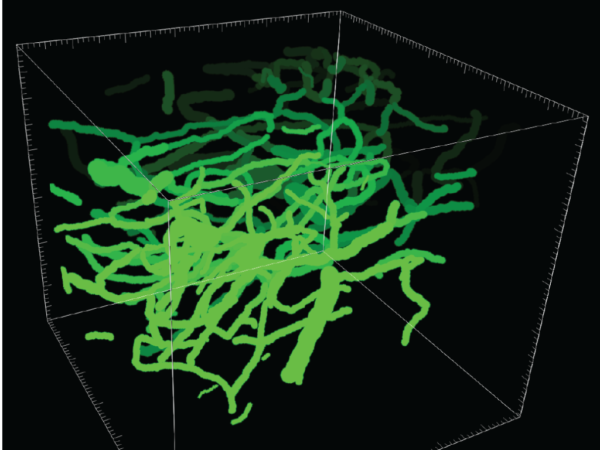
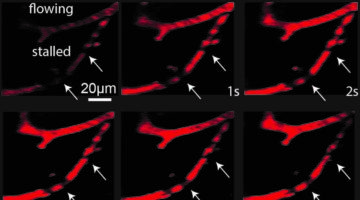
Determine the mechanisms of capillary stalling and brain flow reductions in mouse models of ftd
We have recently shown that Neutrophils adhere to cortical capillaries and contribute to the overall brain blood flow reduction in mouse models of Alzheimer’s disease. Here, we investigate if capillary stalling is contributing to brain blood flow reductions seen in Frontotemporal dementia (FTD) and Parkinson’s disease mouse models. We will specifically address the cause and consequences of brain blood flow reduction associated with these diseases. To achieve this goal, we will perform in vivo multiphoton microscopy combined with an antibody labeling strategy to target the blood cell-types and cells of the neurovascular unit that are involved in adhesion receptor-ligand interaction. In addition to functional deficits in these diseases, structural derangement of the brain microvascular network may also be important. This work will elucidate the structural vascular differences in FTD and Tau mouse models using custom-made analysis tools. This allows the quantification of different matrices, including total length, number of branches, overall vessel density, tortuosity, other vascular obstructions, and sex differences using computational methods. If our hypotheses hold true and we can detect similar vascular deficits in these disease models, this work could explain a common mechanism that contributes to blood flow reductions in neurodegenerative diseases. Later the lab will focus on individual diseases to detriment the cellular cause and molecular mechanisms contributing to CBF reductions in these mice.
Elucidate the role of vascular obstruction following an ischemic stroke in a mouse model of Alzheimer’s Disease
The incidence of stroke prior in life is associated with a higher prevalence of Alzheimer’s Disease. Here we develop a novel mouse model that introduces the incidence of small strokes (micro lesions) in mouse models of Alzheimer’s disease and study their contribution to brain blood flow reductions and the progression of the cognitive impairment associated with stroke. One major question is why do patients affected by a stroke often recover from their motor-sensory deficits, whereas the stroke-associated cognitive decline remains present. It is not well understood why stroke patients have a higher risk to develop Alzheimer’s Disease later in life, a phenomenon called Post-stroke dementia. This research branch will utilize Alzheimer’s Disease mice disease models that have received a micro-lesion (and larger ischemic lesions) early in life, and follow these longitudinally using in vivo multiphoton imaging, magnetic resonance imaging (MRI), behavior testing for short-term memory, motor-sensory function, and molecular analyses of micro-vessels to determine the impact of an ischemic lesion on Alzheimer’s Disease. In an initial, we will establish a new mouse model that allows for long-term imaging in mice that have received a stroke early in their life and measure the progression and severity of vascular dysfunction, brain blood flow reductions, and cognitive impairment in aged healthy mice and mouse models of neurodegenerative disease.
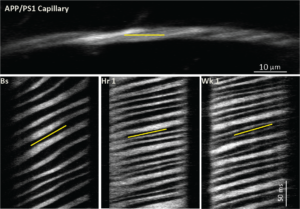
ImprovE Cerebral Blood Flow in mouse models
This research direction will elucidate the molecular mechanism underlying capillary stalling in mouse models of AD. One upstream candidate pathway is the CDC36-NOX2 pathway, which causes vascular inflammation. NOX2 is found in neurons, neutrophils, and, interestingly, in brain endothelial cells, and is a major source of reactive oxygen species in the brain endothelium. Thus, NOX2 contributes to vascular inflammation, a phenomenon that likely underlies the increased neutrophil adhesion we discovered. Furthermore, in transgenic AD mice (Tg2576), Nox2 knockout rescued amyloid plaque-associated dysfunction in neurovascular coupling; and in human AD patients, NOX2 is activated in brain vessels. Recently, we obtained preliminary data indicating that NOX2 inhibition leads to a significant CBF increase in APP/PS1 treated with the NOX2 inhibitor compared to control APP/PS1 mice. The first set of experiments proposed here explores the putative role of this pathway as a mediator of vascular inflammation contributing to CBF reductions capillary stalling. In addition, my lab will analyze the transcriptome of microvessels from APP/PS1 mice treated with a NOX2 inhibitor, and elucidate the transcriptome and proteome of the microvasculature from AD and control mice.